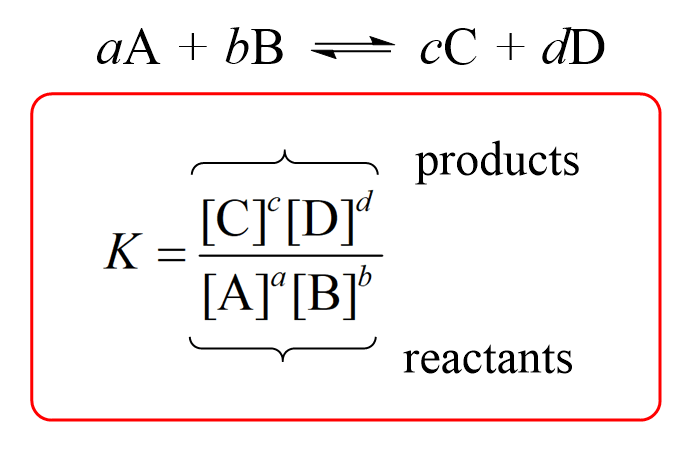
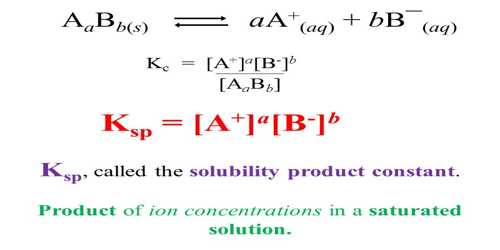
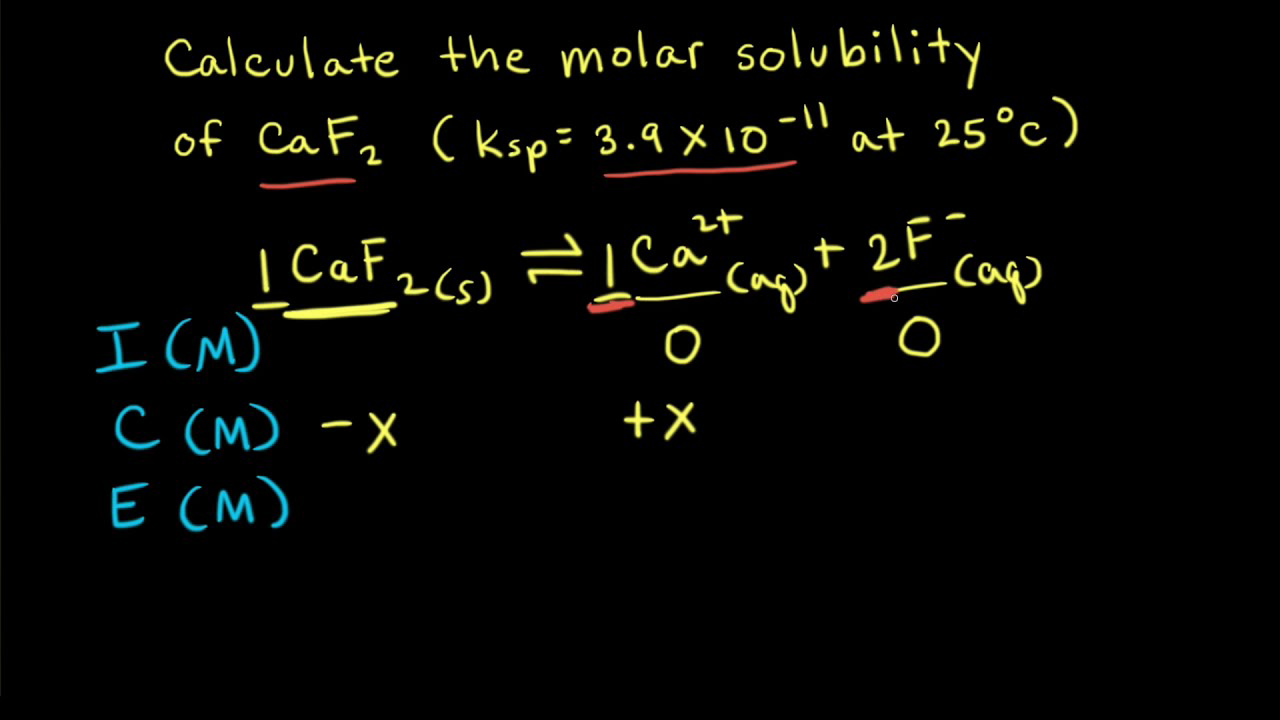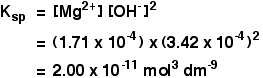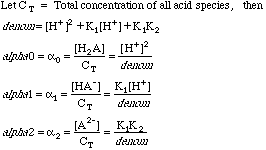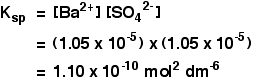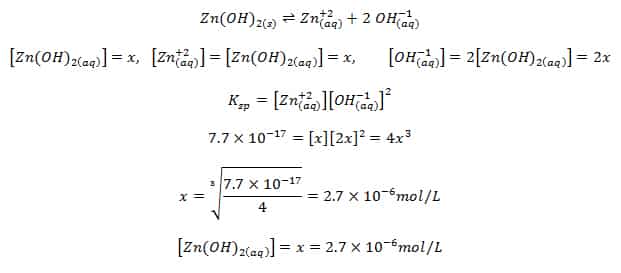The solubility product of Al(OH)3 is 2.7 × 10^-11 . Calculate its solubility in gL^-1 and also find out pH of this solution. (Atomic mass of Al = 27u ).
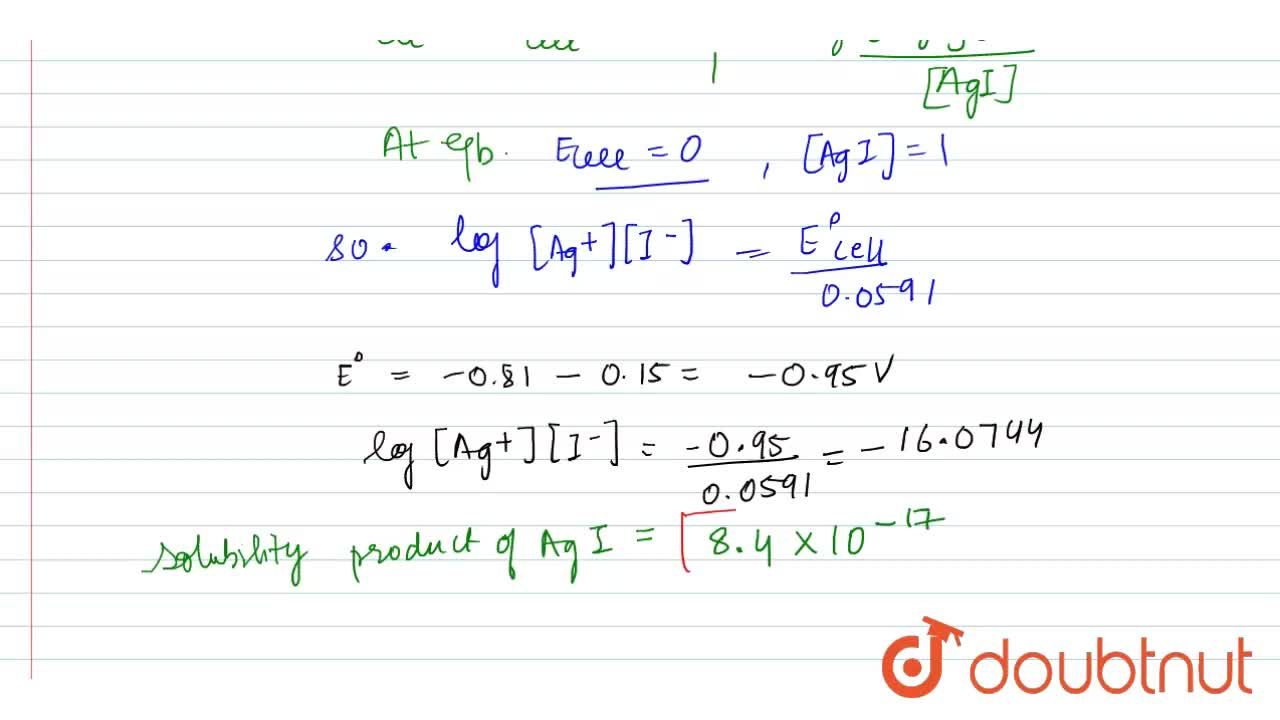
Calculate the solubility product constant of AgI from the following values of standard electrode potentials. E(Ag^(+)//Ag)^(@)=0.80 volt and E(I//AgI//Ag)^(@)=-0.15 volt at 25^(@)C
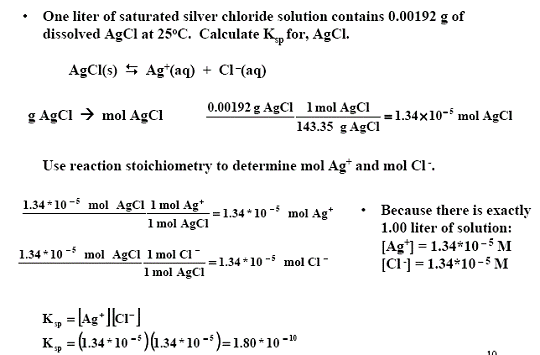
Calculate the solubility product of AgCl if the solubility of the salt in saturated solution at 298 K is 0.00198 g L^(-1).
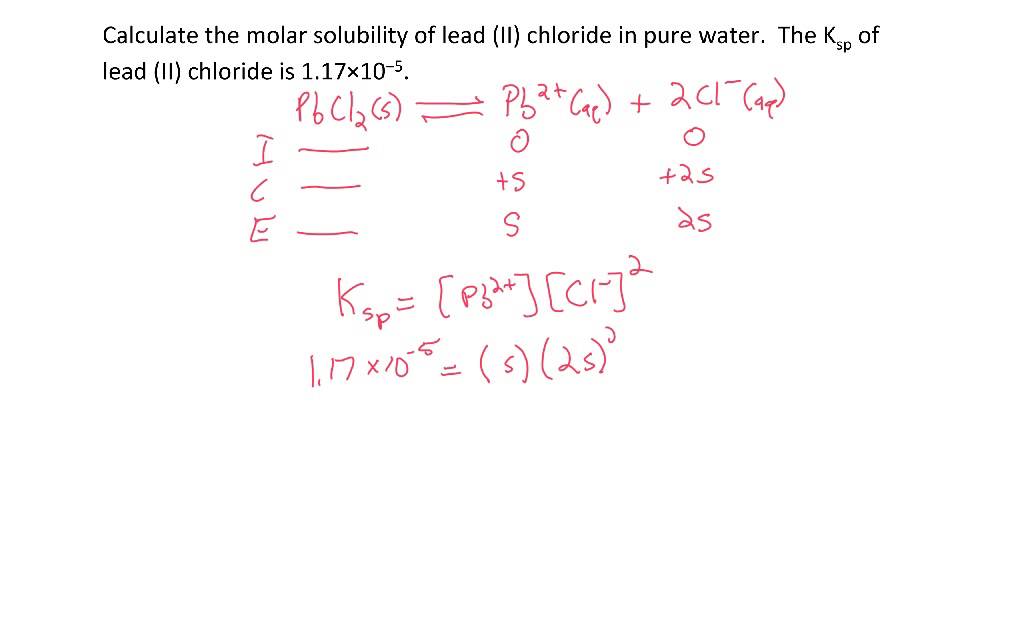
![The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ] The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SFkyRXpPWTdqMnc=/sd/)
The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ]
![SOLVED: 4. Solubility product of AgzSO4 i8 1.5 x 10 - . Using the expression for the value of the solubility product Ksp [Agt] 2 [SO42-] calculate solubility s of AgSO4 ink0.10 SOLVED: 4. Solubility product of AgzSO4 i8 1.5 x 10 - . Using the expression for the value of the solubility product Ksp [Agt] 2 [SO42-] calculate solubility s of AgSO4 ink0.10](https://cdn.numerade.com/ask_images/e0d27084c89e44e09bb144552a2ddaf7.jpg)