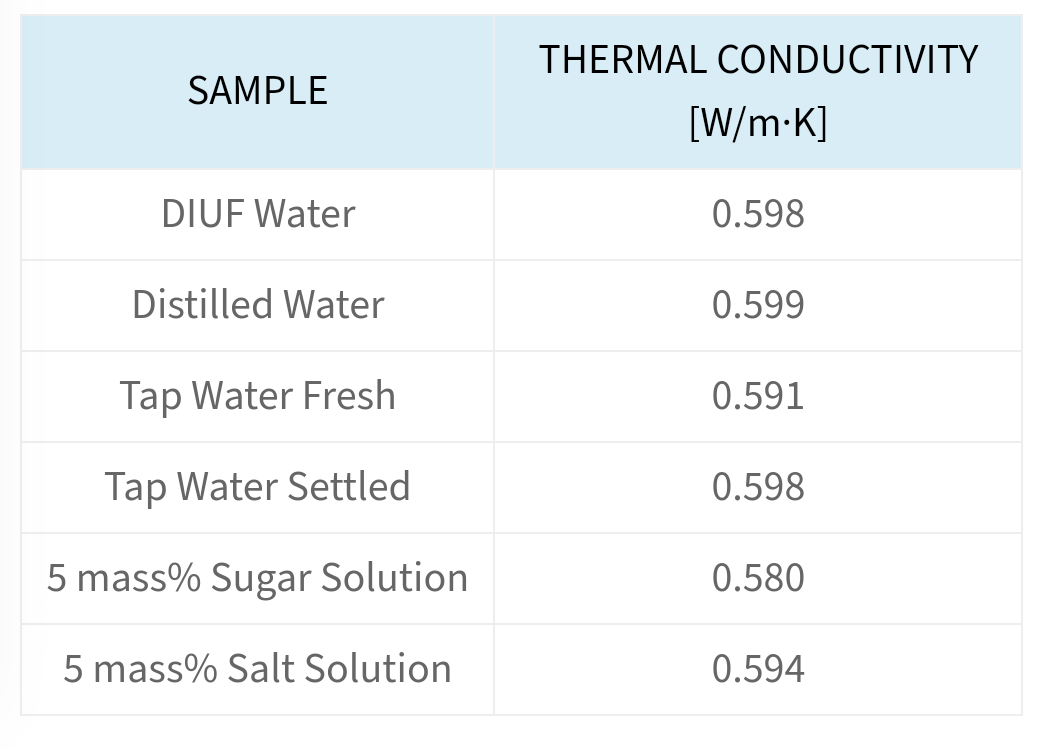
At certain temperature the conductivity of pure water is 6.61/100000000ohm cm the ionic conductance of H+ and OH ions at this temperature are 350 and 200ohm 1 cm2 mol 1 respectively the
The conductivity of water at 298K is 0.55 × 10^ 7 S cm^ 1. if λ m(H^+)=350 S cm^2 mol^ 1 and λ(OH^ )=200 S cm^2, the degree of dissociation of water will b

Specific conductance of pure water at `25^(@)C` is `0.58 xx 10^(-7)` mho `cm^(-1)`. Calculate - YouTube








.webp)

![Water resistivity and conductivity at 25 °C [3, 4] | Download Scientific Diagram Water resistivity and conductivity at 25 °C [3, 4] | Download Scientific Diagram](https://www.researchgate.net/publication/267975875/figure/tbl1/AS:941098180550661@1601386699176/Water-resistivity-and-conductivity-at-25-C-3-4.png)