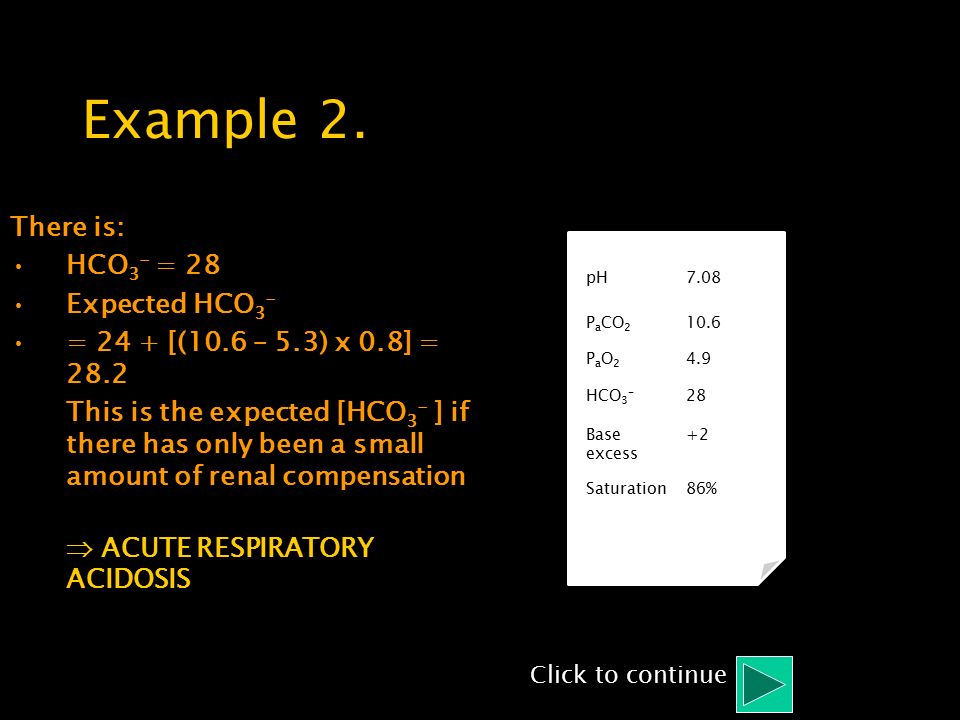
![PDF] Discrepancy between Measured Serum Total Carbon Dioxide Content and Bicarbonate Concentration Calculated from Arterial Blood Gases | Semantic Scholar PDF] Discrepancy between Measured Serum Total Carbon Dioxide Content and Bicarbonate Concentration Calculated from Arterial Blood Gases | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d604591e54c0e843b180644b6e556990864a0b37/7-Figure4-1.png)
PDF] Discrepancy between Measured Serum Total Carbon Dioxide Content and Bicarbonate Concentration Calculated from Arterial Blood Gases | Semantic Scholar

Can someone explain where did the CO3(2-) and HCO3(-) came from? And how do I write the equations with HCl and Na2CO3? : r/chemhelp
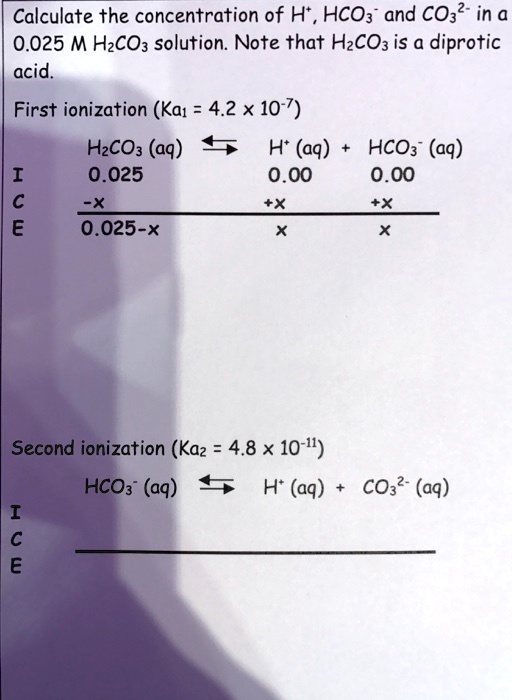
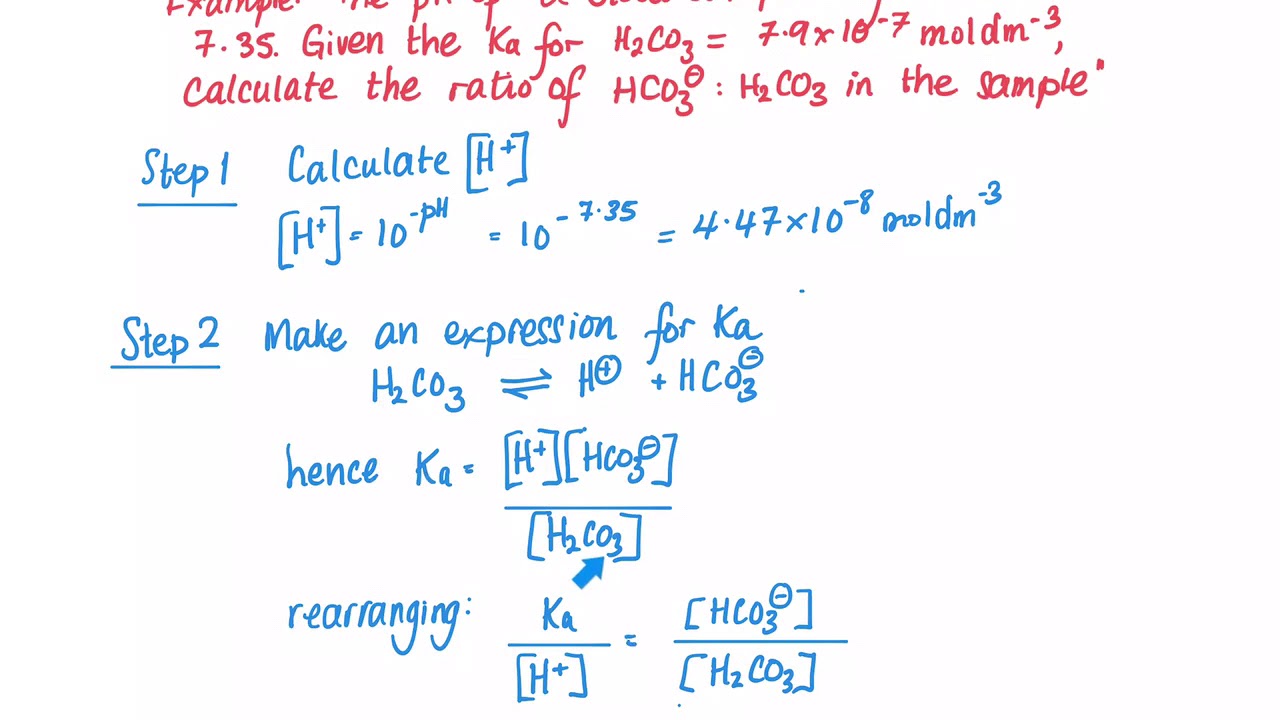
SOLVED: Calculate the concentration of H+, HCO3-, and CO3^2- in a 0.025 M H2CO3 solution. Note that H2CO3 is a diprotic acid. First ionization (Ka1 = 4.2 x 10^-7): H2CO3 (aq) ->

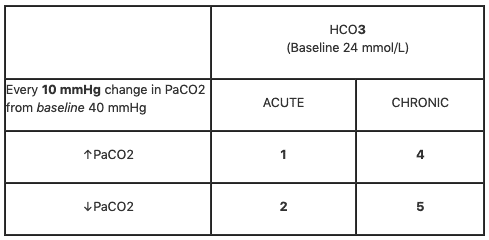
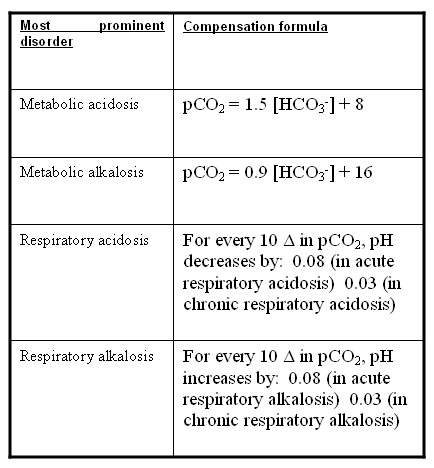
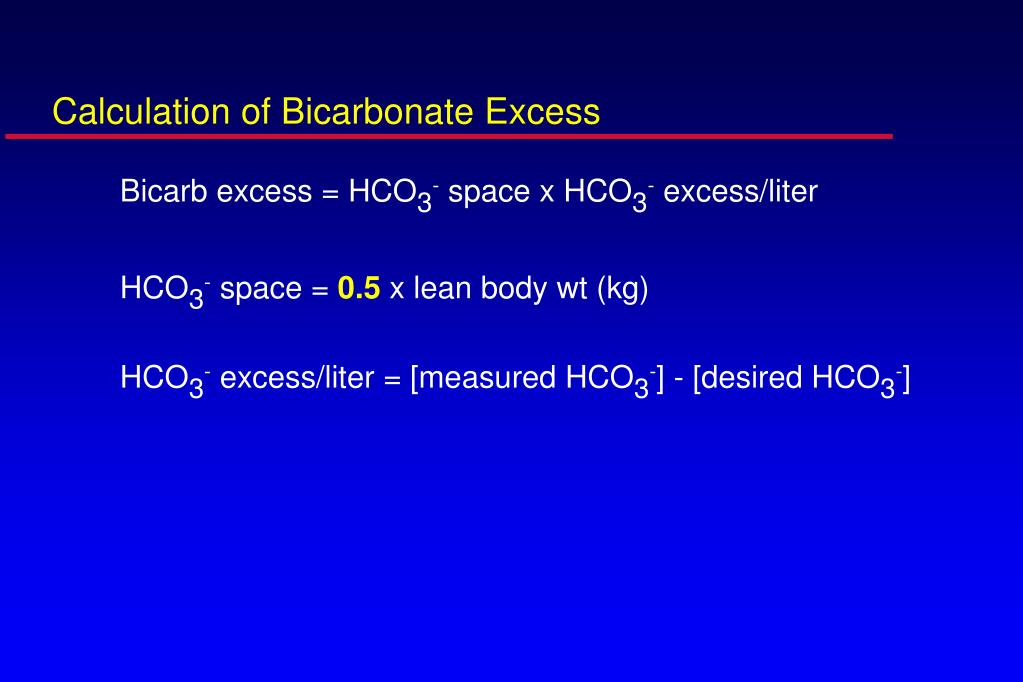
Rishi Kumar, MD - I created this table to teach my trainees how I approach acid-base problems assuming a normal bicarbonate (HCO3) of 24 mmol/L, PaCO2 40 mmHg, arterial pH 7.35-7.45, and
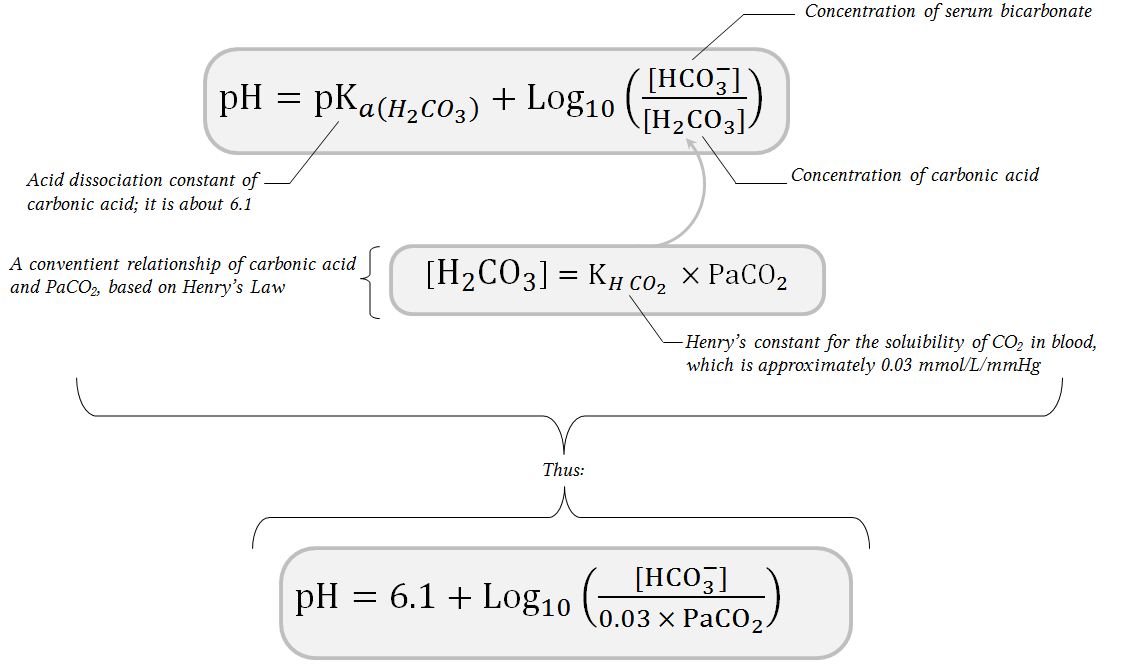
![SOLVED: The concentration of bicarbonate is calculated using the equation below. What is the concentration of bicarbonate for a pH of 7.0 and a PaCO2 of 40 mmHg? [HCO3] pH = 6.1 + SOLVED: The concentration of bicarbonate is calculated using the equation below. What is the concentration of bicarbonate for a pH of 7.0 and a PaCO2 of 40 mmHg? [HCO3] pH = 6.1 +](https://cdn.numerade.com/ask_images/6f18db30c9624fa99fdc4cda7c2f7592.jpg)
SOLVED: The concentration of bicarbonate is calculated using the equation below. What is the concentration of bicarbonate for a pH of 7.0 and a PaCO2 of 40 mmHg? [HCO3] pH = 6.1 +
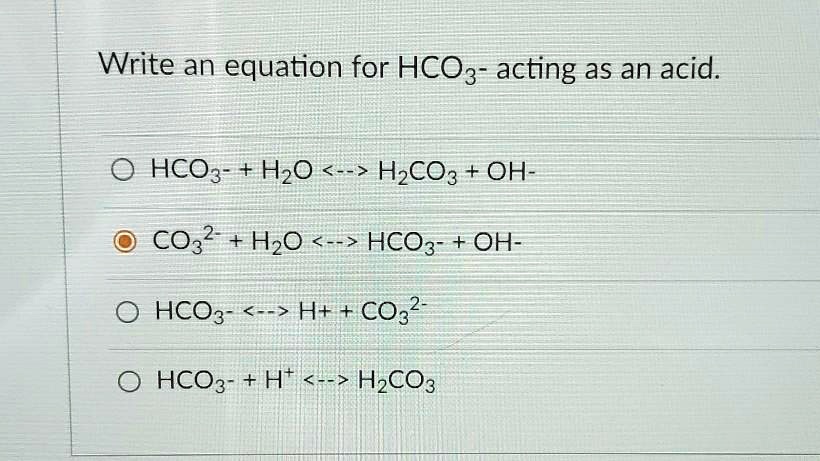
SOLVED: Write an equation for HCO3- acting as an acid: HCO3- + H2O <-> H2CO3 + OH- CO3 + H2O <-> HCO3- + OH- HCO3- <-> H+ + CO3^2- HCO3- <-> H2CO3

SOLVED: The pH of human blood is maintained at7.40 and is primarily controlled by the carbonic acid/bicarbonate (HCO/HCO)acid-base system.Use the HH equation to calculation the HCO3concentration if the HCO3 concentration is 24.5
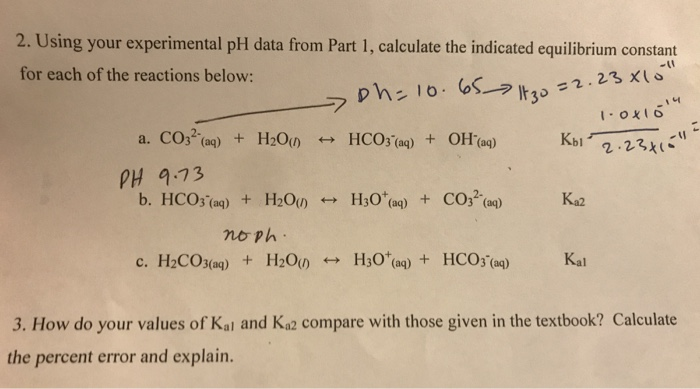
![Solved Calculate the value of the ratios [HCO3-]/[CO32-] | Chegg.com Solved Calculate the value of the ratios [HCO3-]/[CO32-] | Chegg.com](https://media.cheggcdn.com/media/0b6/0b6129fb-dc7d-45f2-9788-1b56098b9225/phprZKjss)