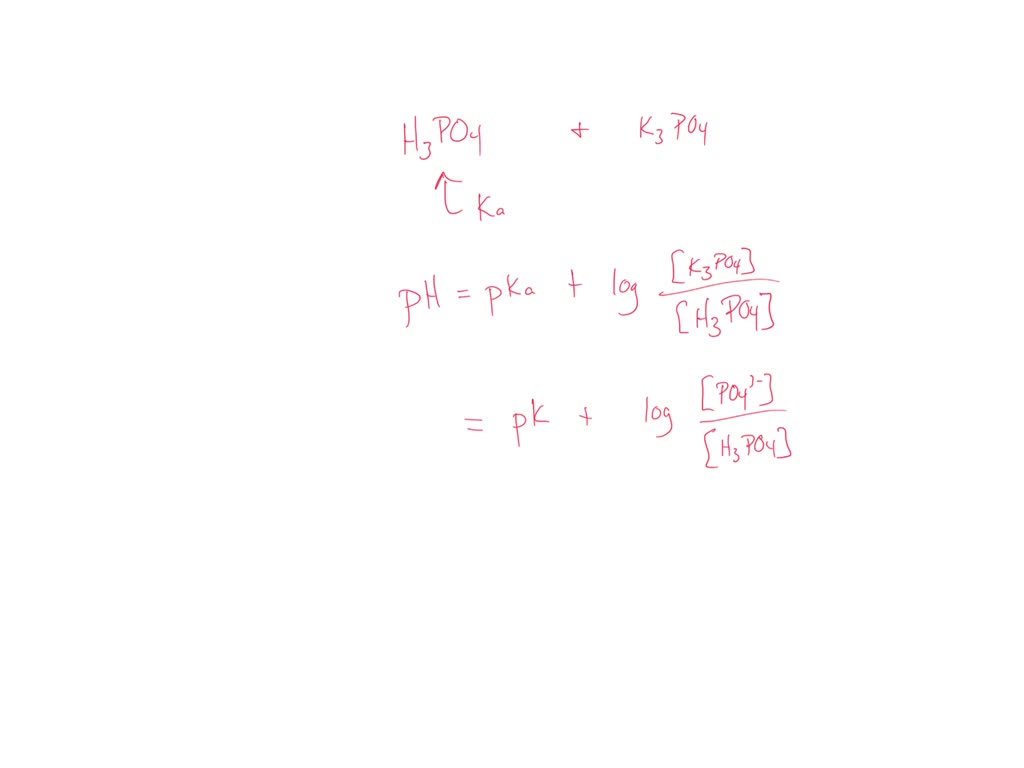Preparation of Buffers - 1 Calculate the volume of sulfuric acid (H 2 SO 4 ) necessary to prepare 600 milliliter 0.5M H 2 SO 4 from concentrated H 2 SO. - ppt download

Mobile Phase Buffers in Liquid Chromatography (LC): Effect of Buffer Preparation Method on Retention Repeatability

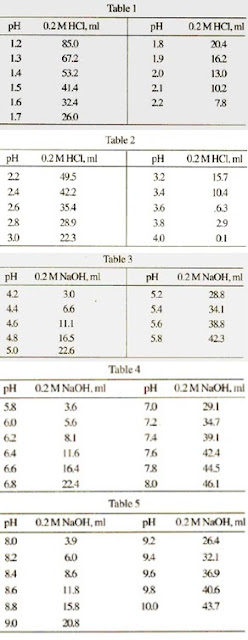
SOLVED: You need to prepare 100 mL of a 0.045 M Potassium phosphate buffer ( pH 6.8). Calculate the % base in your buffer. Round your answer to two decimals. Jy moet 100

velocities were obtained in potassium phosphate buffer at 30 mC, pH... | Download Scientific Diagram